IMPAK Gets FDA Clearance

FDA Cleared Comfort & Performance
We're thrilled to announce a breakthrough moment for dental comfort: IMPAK – our revolutionary thermoresponsive acrylic material – has officially received FDA clearance!

IMPAK is an acrylic material with unique physical properties, characterized by softening when heated and returning to its original hard state when cooled.
This characteristic makes IMPAK more comfortable to wear inside the mouth than other materials.
It's mainly used for the production of bite splints, night guards and sport guards.

Why This Changes Everything
For decades, patients have struggled with rigid, uncomfortable oral appliances. IMPAK solves this with biomimetic intelligence:Start softening at body temperature (37°C/98.6°F) like memory foamRe-hardens when cooled for durable functionalityCreates a truly custom-fit experience in seconds
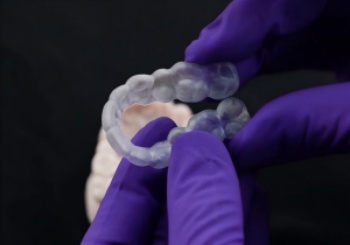
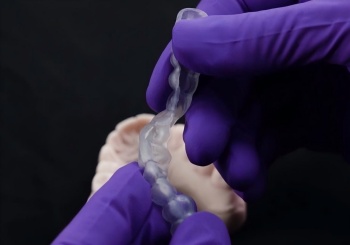
*It has a better softening effect at 60 degrees (140 °F).
Clinical Benefits You Can't Ignore
1. Easy operation and high efficiency
· Suitable for CAD/CAM
· Matched with various types of CAD/CAM equipment, with multiple sizes
2. High patient comfort
Softens with heat and has good intraoral adaptability
· Softens with heat and has good intraoral adaptability
· High biological safety, MMA-free
3. Excellent performance
· High tear resistance and stability
· Low water absorption and dissolution
4. Clinical economy

· Simplify the process, reduce personnel manual operation
· Low cost, less waste of consumables
Better for Patients, Easier for You!
With IMPAK, patients enjoy soft, comfortable wear with less bulk, less pressure, and a more natural feel – all while keeping the durability you need.
Click here for clinical data and more information!
 English
English  日本語
日本語  français
français  Deutsch
Deutsch  Español
Español  русский
русский  português
português 


